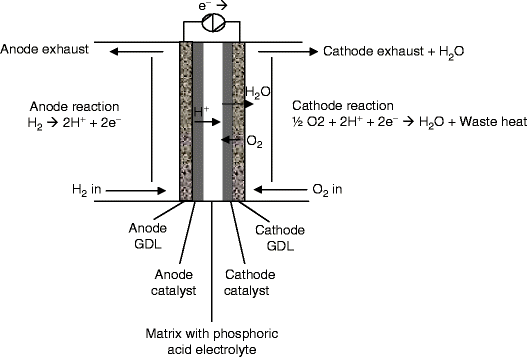
Function and reactions of pafcs.
Pafc fuel cell reaction.
Protons h are transported from the anode to the cathode.
The overall fuel cell reaction is 2h 2 o2 2h 2 o heat.
This paper presents the dynamics of a phosphoric acid fuel cell pafc and its associated power electronics.
They were the first fuel cells to be commercialized.
A substantial number of pafc systems fueled with natural gas are already in operation around the world.
Phosphoric acid fuel cell pafc a type of fuel cell in which the electrolyte consists of concentrated phosphoric acid h 3 po 4.
The pafc differs from other fuel cell technologies mainly on the basis of the electrolyte used and the method of hydrogen generation for the cell reaction.
Excess airflow on the cathode side helps to remove the water resulting from the reaction.
Proton exchange membrane fuel cell pem.
The gas diffusion electrodes are composed of a porous substrate carbon or cloth facing the gas feed and a reactive catalyst layer consisting of platinized fine carbon powder facing the electrolyte.
Unlike other fuel cell technologies pafc is very resistant to poisoning by carbon monoxide and the fuel cell is able to operate with lower hydrogen and oxygen quality.
The mcfc works at a relatively high temperature higher than 600 c.
Hydrogen is able to permeate the phosphoric acid while electrons are not.
Owing to the low temperature operation faster response to load transients and short start up time less than a minute the pemfc is suitable for propulsion applications.
Developed in the mid 1960s and field tested since the 1970s they have improved significantly in stability performance and cost.
Pafcs use liquid phosphoric acid as an electrolyte and run on hydrocarbon fuel.
Liquid phosphoric acid pa dispersed in a silicon carbide matrix acts as the electrolyte.
The reaction process is similar to pemfcs with hydrogen being removed from the fuel via the assistance of a platinum catalyst.
A phosphoric acid fuel cell pafc is composed of two porous gas diffusion electrodes namely cathode and anode juxtaposed against a porous electrolyte matrix.
Molten carbonate fuel cells mcfc.
The operating temperature range is generally 160 220 c.
The resultant reaction is 2h o2 2e h 2 o.
They are quite resistant to poisoning by carbon monoxide but tend to have lower efficiency than other fuel cell types in producing electricity.
The modeling of the power conditioning system for phosphoric acid fuel cell is discussed.